Latest Research News Detail
Nb2O5・nH2O as a heterogeneous catalyst with water-tolerant Lewis acid sites
Summary
The details of research
Nb2O5・nH2O as a heterogeneous catalyst with water-tolerant Lewis acid sites
Lewis acids function as catalysts for the formation of C?C bonds in organic compounds, and therefore the chemical industry utilizes large amounts of Lewis acid catalysts?such as AlCl3, BF3, and transition metal halides?for the production of industrially important chemicals.
However, these homogeneous catalysts decompose or are ineffective in water, an environmentally benign solvent, and have serious drawbacks such as the production of waste, hazards of handling, separation from products, and corrosion of equipment. In addition, the recovery and reuse of homogeneous Lewis acid catalysts is an extremely formidable task.
Now, Michikazu Hara and colleagues at Tokyo Institute of Technology have demonstrated that Nb2O5・nH2O, a stable and insoluble solid, has Lewis acid sites workable even in water.
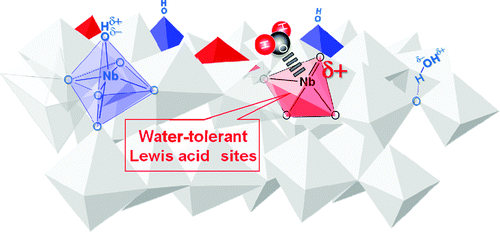
Nb2O5・nH2O is composed mainly of distorted NbO6 octahedra and NbO4 tetrahedra. While NbO4 tetrahedra function as Lewis acid sites, they have been considered not to function in water. However, spectroscopic analyses suggest that NbO4 tetrahedra can react with acidic molecules even if water molecules are attached to them.
In fact, Nb2O5・nH2O efficiently catalyzes Lewis acid-catalyzed reactions in water such as allylation and conversion of glucose into 5-(hydroxymethyl)furfural (HMF). Conversion of glucose, a key component of cellulosic biomass, into HMF is an attractive alternate route to sustainable chemical production; HMF can be further converted into various polymers, synthetic rubbers, plastics and pharmaceuticals.
The Lewis acid sites workable in water are due to NbO4 tetrahedra that still have effective positive charges as Lewis acid sites even after the formation of NbO4?H2O adducts.
Reference:
Authors: Kiyotaka Nakajima, Yusuke Baba, Ryouhei Noma, Masaaki Kitano, Junko N. Kondo, Shigenobu Hayashi, and Michikazu Hara
Title of original paper:Nb2O5・nH2O as a Heterogeneous Catalyst with Water-Tolerant Lewis Acid Sites
Journal, volume, pages and year: Journal of The American Chemical Society 133, 4224, 2011. Digital Object Identifier (DOI): 10.1021/ja110482r
Affiliations: Materials and Structures Laboratory
Department website: http://www.msl.titech.ac.jp/index.html
Structure of Nb2O5・nH2O.
| Reference |
Michikazu Hara
Solutions Research Labolatory Professor |
|---|---|
| TEL | |
| FAX | |
| URL |