Latest Research News Detail
Polysilane rings: Selective cyclopolymerization using transition...
Summary
The details of research
Polysilane rings: Selective cyclopolymerization using transition metals
Polysilanes, composed of the Si?Si linkage, exhibit unique electronic and optical properties that result from the extensive delocalization of σ-electrons along the polymer backbones.
Transition-metal-catalyzed dehydrocoupling polymerization of organosilanes is the most common method for the synthesis of polysilanes. Early transition metals such as titanium and zirconium are effective catalysts to provide the linear polysilanes, while use of late transition metals (rhodium, palladium, etc.) have been much rarer than the early metals.
Now, Makoto Tanabe and his colleagues at Chemical Resources Laboratory of Tokyo Institute of Technology have found that a nickel complex is very effective for dehydrocoupling reactions of organosilanes, forming the cyclic polysilanes selectively.
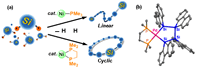
The Ni/PMe3 catalytic system, prepared in situ, led to polycondensation of phenylsilanes to produce the linear polysilanes, similar to the results catalyzed by early transition metals. Similar reactions using a ![]() system resulted in selective cyclopolymerization to yield cyclic polysilanes involving the 9-11 silicon units. Formation of a palladium tetrasilane ring complex implies it being a possible intermediate for formation of the cyclic polymers.
system resulted in selective cyclopolymerization to yield cyclic polysilanes involving the 9-11 silicon units. Formation of a palladium tetrasilane ring complex implies it being a possible intermediate for formation of the cyclic polymers.
This study demonstrates the first controlled polymerization to produce linear or cyclic polysilanes by the appropriate choice of catalysts. The cyclic polysilanes might be interested in exhibiting the unique properties attributed to the cyclic conjugated structure.
Reference:
- Authors: Makoto Tanabe, Atsushi Takahashi, Tomoko Fukuta, Kohtaro Osakada
- Title of original paper: Nickel-Catalyzed Cyclopolymerization of Hexyl- and Phenylsilanes
- Journal, volume, pages and year: Organometallics 32, 1037 (2013).
- Digital Object Identifier (DOI): 10.1021/om301052f
- Affiliations: Chemical Resources Laboratory, Tokyo Institute of Technology
Department website:http://www.res.titech.ac.jp/~shinkin/
Fig. 1. (a) Nickel-catalyzed polymerization to afford linear or cyclic polysilanes and (b) one of possible intermediates in the cyclopolymerization.
| Reference |
Makoto Tanabe
Chemical Resources Laboratory Assistant Professor |
|---|---|
| TEL | |
| FAX | |
| URL |